Concentration of Ores
Concentration of Ores: Overview
This topic covers concepts such as Leaching of Silver, Concentration or Dressing of Ores, Froth Floatation Process, Gravity Separation or Levigation, Magnetic Separation Method, Frothing Agent, Collectors, Depressants, Froth Stabilizers, etc.
Important Questions on Concentration of Ores
Match the following processes of metallurgy with their corresponding ore for which they are used:
(i) Froth floatation method (a) Germanium
(ii) Electrolytic refining of metals (b) ZnS
(iii) Zone refining of metals (c) Copper
In the process of extraction of gold,
An ore of tin containing is concentrated by
Give an example of collectors.
What is the function of collectors in froth floatation process?
The oil used as frothing agent in froth flotation process is
The addition of gold or silver to an alkaline sodium cyanide solution will cause the metals to react with the cyanide and dissolve them into the solution. The process is called
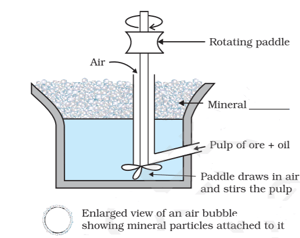
The most appropriate term for the blank in the image below is (The image below is the process of removing gangue from sulphide ores):
What is in the following reaction?
Out of and (ores of lead), which one is concentrated by froth floatation process preferably?
What is the role of depressants in the froth floatation process?
Sulphur is extracted by_____.
Write the name of two impurities present in bauxite.
What is the use of collectors in froth floatation process?
Which of the following compounds are used in froth floatation process?
Which of the following ores are concentrated by froth flotation?
Complete the following reaction-
+ .
Complete the following reaction-
+ .
In the formation of and for formation of , , will it be possible that is reduced by . Give reasons.
Describe the Froth- Flotation process.